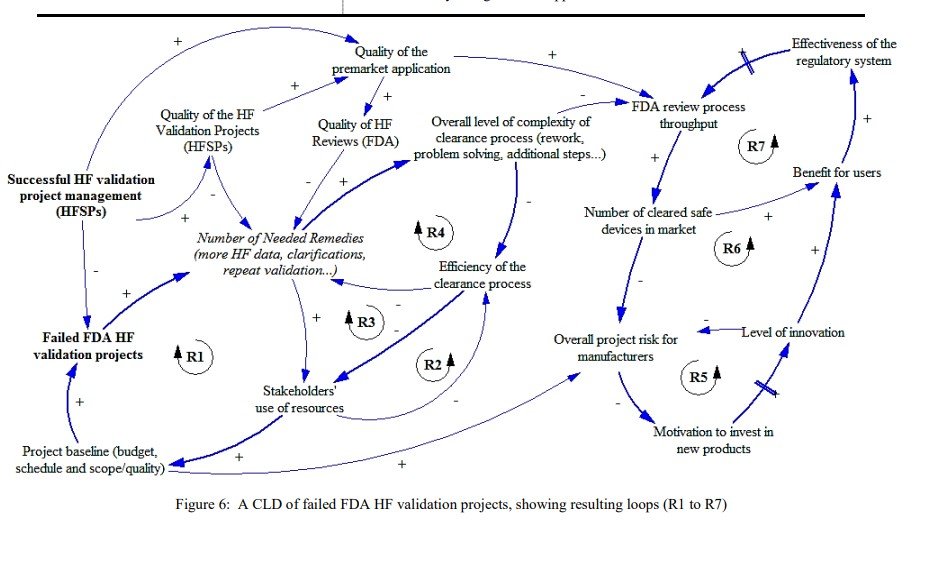
Recognizing the role of human factors engineering (HFE) in the development of medical devices and combination products that involve devices, the Food and Drug Administration (FDA) now requires human factors (HF) validations before market approval. Manufacturers are responsible for ensuring their products are safe and effective through the application of HFE. However, key stakeholders are still learning and developing capabilities to adapt to the regulatory component. Nonetheless, the lack of the corresponding HF capabilities hinders compliance with the FDA’s expectations, and thus ultimate success. No known previous work has looked into FDA HF validation projects to assess the underlying factors and implications of failed submissions. Applying system dynamics (SD), a causal loop diagram (CLD) was developed. CLDs are useful for the exploration of the causal interactions among factors or variables, as well as the underlying feedback structure of a complex system. This work can serve to help manufacturers better understand the FDA’s HF requirement to enable overall product success. Further, with patient safety as a common goal, HF service providers (HFSPs) and regulators should be aware of the need to ensure the consistent quality of the HF element in premarket submissions.
Considering the Dynamics of FDA Human Factors Validation Requirement: Implications of Failure and Need to Ensure Project Success– A Conceptual Framework
Was this helpful?
Thanks for your feedback!