Go Beyond Regulatory Standards Effortlessly
Bridge the Gap: Advancing Human Factors Maturity in MedTech
Discover the potential of the groundbreaking maturity framework, SHF 2.0. Developed through scientific research and designed to carefully align medical HF with standards (e.g.: ISO 13485, ISO 14971), essential guidelines and key success factors, it drives best practices for user-centered product development while seamlessly meeting regulatory expectations!
Ready to leverage this innovative framework?
Addressing Critical Needs
Behind Schedule
All manufacturers experience delays in completing HF projects, and a significant 70% of external HF service providers or consultancy firms encounter similar challenges.
No Quality System
Half of HF service providers lack a quality system, show unawareness or have no plans to implement any kind of systematic approach for ensuring HF project quality.
Failure Rate
According to FDA data, failure rates per HF submission type: 96.1% for Q-sub, 93.5% for PMA, and 89.5% for 510(k).
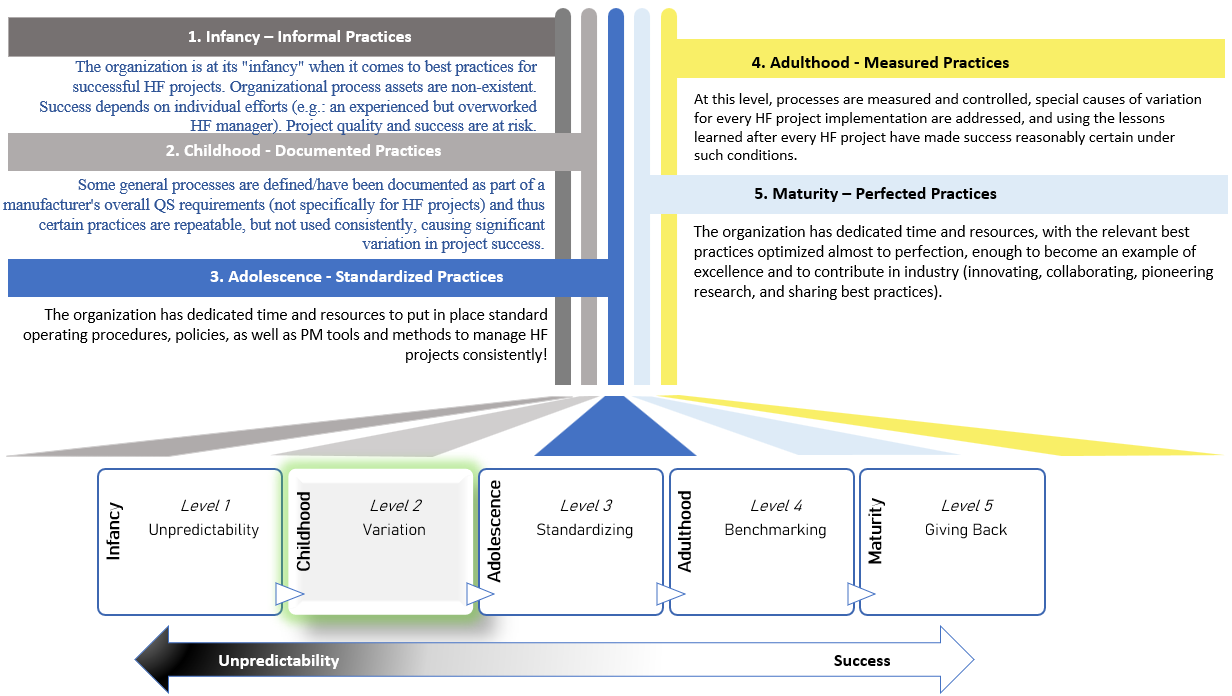
Get to Know the SHF 2.0 Framework
Rise above suboptimal outcomes and embrace consistent HF excellence
SHF 2.0 uniquely enables integration and streamlined approach that stakeholders demand in today’s ever-evolving landscape. From human factors supplier audits to strategic project management, SHF 2.0 is the trailblazer that positions you ahead of the game.
By harnessing SHF 2.0, teams can effectively mitigate risks, reduce errors, and expedite project outcomes. The framework goes beyond regulatory requirements, it enables organizations to harmonize procedures, cultivate a culture of quality, and unlock the full potential of HF. Embrace the future of HF excellence with the proven, industry-focused SHF 2.0 framework and go beyond regulations effortlessly!
Know your Successful HF Capability?
Rise above suboptimal outcomes and embrace consistent HF excellence.
Use our quick and easy quiz and find out how you stack across the emerging best practices, organized in 5 categories as per the SHF 2.0 framework.
More! Get access to the current benchmark report and find out how you are doing in the adoption of the emerging best practices for successful HF projects in the medical device industry.
Original Insights

The Future of AI in Healthcare Development: Successful Human Factors™ Adds AI Integration Services Powered by Pioneering AI Copilot Suabix™ (Now Live!)

Original Insights: Success Factors of Human Factors Validation in Medical Device Development

Navigating the Shadows: A Tale of Innovation, Plagiarism, and the Battle for Ethical Standards in Human Factors and Usability Engineering

Announcement: Introducing Suite of Services to Support Healthcare Teams with Innovative Solutions!

From Research to Policy: Exploring Precedents of HFES Maturity Framework Policy Recommendations to the FDA

Suabix: AI-Powered Human Factors Innovation, Trademark Filed
Introducing Suabix™ by Successful Human Factors™ - First AI Solution to Optimize and Standardize Human Factors in Medical Device Design





