Developing an Industry-Focused Maturity Assessment Tool Based on Key Factors Critical to Quality and Success in FDA Human Factors Validation Projects – Overview
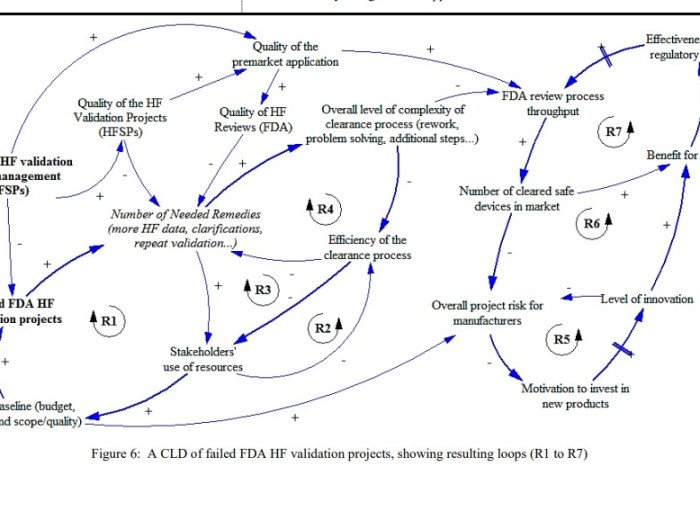
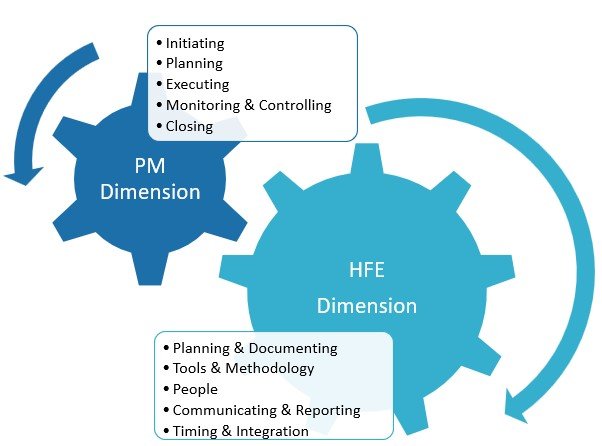
This work is Phase II of a research theme on the topic of human factors validation projects for medical devices and combination products. Initially, a review and analysis of the persisting concerns and also of the implications of failed FDA HF validation projects took place. One main problem delineated was that key stakeholders (namely procurers and […]
Continue reading "Developing an Industry-Focused Maturity Assessment Tool Based on Key Factors Critical to Quality and Success in FDA Human Factors Validation Projects – Overview"