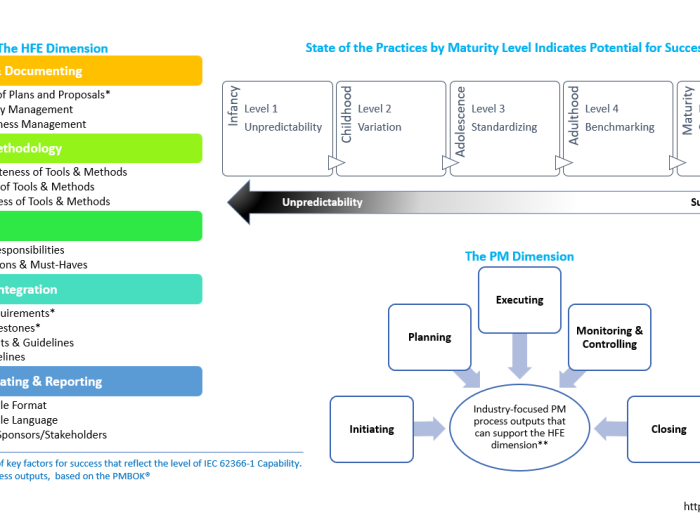
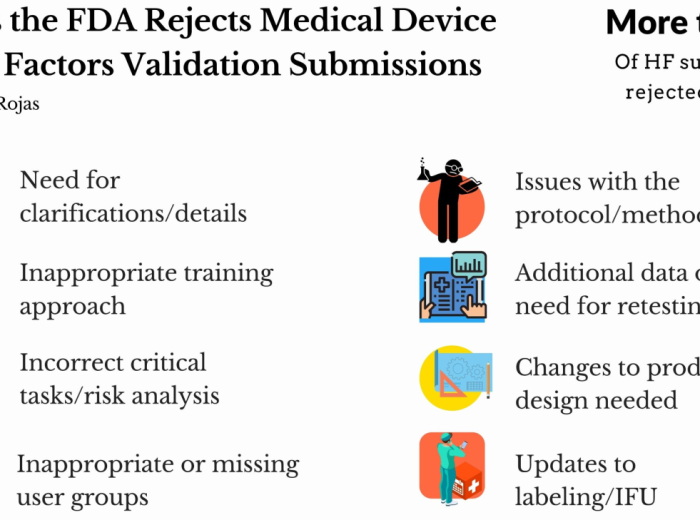
Redefining Human Factors Validations: Strategies to Increase Maturity for Successful Human Factors in Medical Devices and Combination Products
The integration of human factors (HF) engineering (or human-centered design) in the development of medical devices and combination products has become increasingly crucial for ensuring their safety, usability, and overall effectiveness. However, the implementation of HF practices has been accompanied by various challenges, hindering successful validation projects and regulatory compliance. It is clear that traditional approaches […]
Continue reading "Redefining Human Factors Validations: Strategies to Increase Maturity for Successful Human Factors in Medical Devices and Combination Products"