Leveraging the SHF 2.0 Framework to Overcome Common Challenges in Human Factors Validation Projects: A Strategy & Implementation Approach
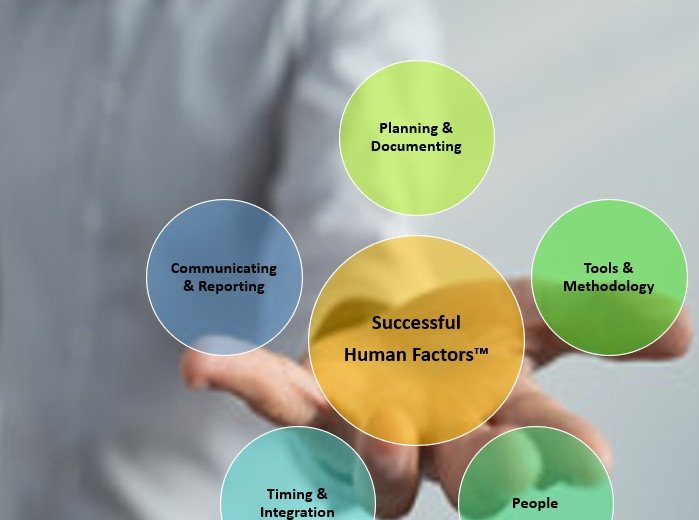
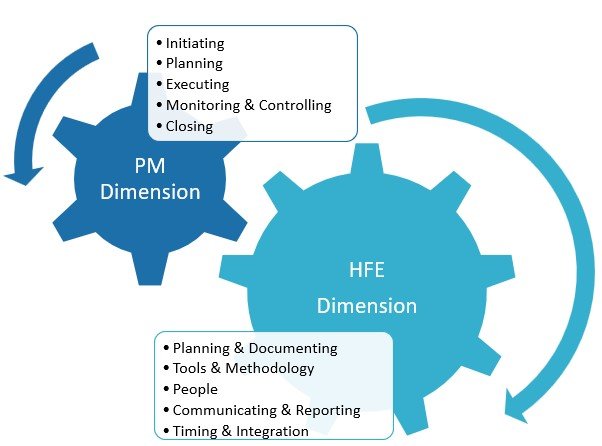
We have previously discovered through research the challenges that Human Factors (HF) Validation projects in the medical device and combination products industry often face – study participant-related, changes to IFU and packaging design, engaging sponsors, and more (Rojas et al., 2020). To effectively address these challenges, organizations can leverage the Successful Human Factors (SHF) 2.0 […]
Continue reading "Leveraging the SHF 2.0 Framework to Overcome Common Challenges in Human Factors Validation Projects: A Strategy & Implementation Approach"