Go Beyond Regulatory Standards Effortlessly
Bridge the Gap: Advancing Human Factors Maturity in MedTech
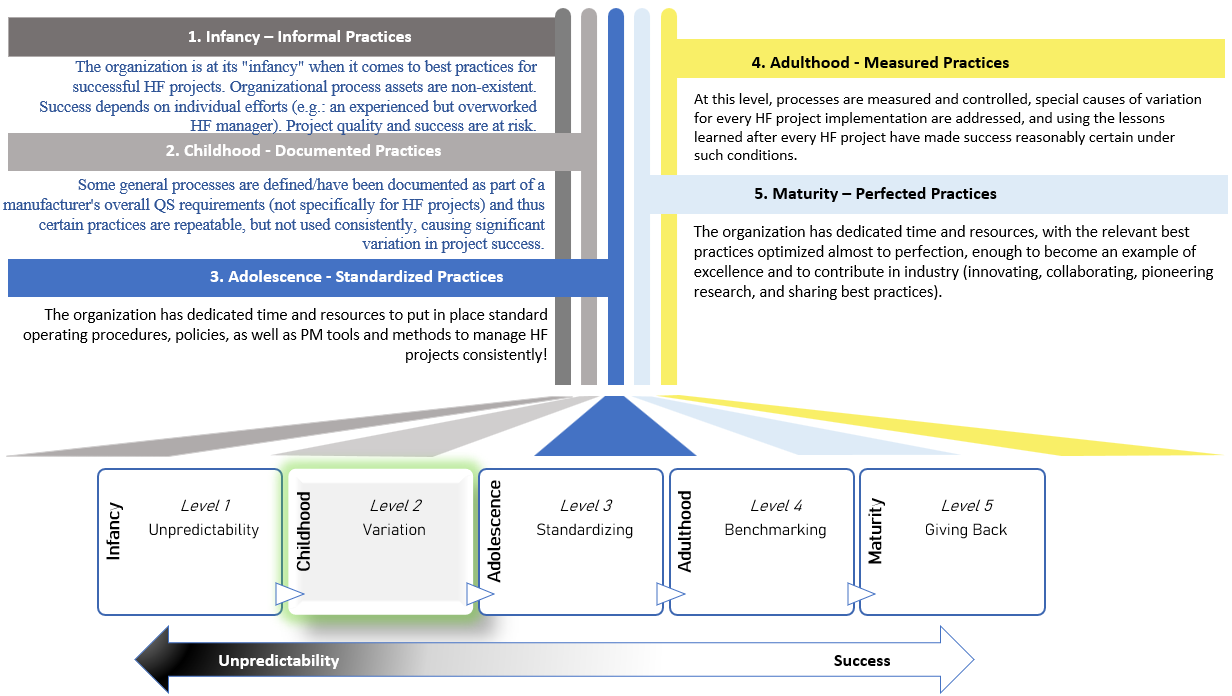
Discover the potential of the groundbreaking maturity framework, SHF 2.0. Developed through scientific research and designed to carefully align medical HF with standards (e.g.: ISO 13485, ISO 14971), essential guidelines and key success factors, it drives best practices for user-centered product development while seamlessly meeting regulatory expectations!
Ready to leverage this innovative framework?
Addressing Critical Needs
Behind Schedule
All manufacturers experience delays in completing HF projects, and a significant 70% of external HF service providers or consultancy firms encounter similar challenges.
No Quality System
Half of HF service providers lack a quality system, show unawareness or have no plans to implement any kind of systematic approach for ensuring HF project quality.
Failure Rate
According to FDA data, failure rates per HF submission type: 96.1% for Q-sub, 93.5% for PMA, and 89.5% for 510(k).
Get to Know the SHF 2.0 Framework
Rise above suboptimal outcomes and embrace consistent HF excellence
SHF 2.0 uniquely enables integration and streamlined approach that stakeholders demand in today’s ever-evolving landscape. From human factors supplier audits to strategic project management, SHF 2.0 is the trailblazer that positions you ahead of the game.
By harnessing SHF 2.0, teams can effectively mitigate risks, reduce errors, and expedite project outcomes. The framework goes beyond regulatory requirements, it enables organizations to harmonize procedures, cultivate a culture of quality, and unlock the full potential of HF. Embrace the future of HF excellence with the proven, industry-focused SHF 2.0 framework and go beyond regulations effortlessly!
Know your Successful HF Capability?
Rise above suboptimal outcomes and embrace consistent HF excellence.
Use our quick and easy quiz and find out how you stack across the emerging best practices, organized in 5 categories as per the SHF 2.0 framework.
More! Get access to the current benchmark report and find out how you are doing in the adoption of the emerging best practices for successful HF projects in the medical device industry.
Original Insights
[{"id":21206,"link":"https:\/\/successfulhf.com\/the-future-of-ai-in-healthcare-ai-integration-services-powered-by-pioneering-ai-copilot-suabix-now-live\/","name":"the-future-of-ai-in-healthcare-ai-integration-services-powered-by-pioneering-ai-copilot-suabix-now-live","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/suabix-2.png","alt":""},"title":"The Future of AI in Healthcare Development: Successful Human Factors\u2122 Adds AI Integration Services Powered by Pioneering AI Copilot Suabix\u2122 (Now Live!)","author":{"name":"Staff","link":"https:\/\/successfulhf.com\/author\/staff\/"},"date":"May 7, 2024","dateGMT":"2024-05-07 00:30:07","modifiedDate":"2024-07-29 22:54:12","modifiedDateGMT":"2024-07-29 22:54:12","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/industry-news\/\" rel=\"category tag\">Industry News<\/a>, <a href=\"https:\/\/successfulhf.com\/category\/suabix\/\" rel=\"category tag\">Suabix AI<\/a>, <a href=\"https:\/\/successfulhf.com\/category\/updates-announcements\/\" rel=\"category tag\">Updates & Announcements<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/industry-news\/\" rel=\"category tag\">Industry News<\/a> <a href=\"https:\/\/successfulhf.com\/category\/suabix\/\" rel=\"category tag\">Suabix AI<\/a> <a href=\"https:\/\/successfulhf.com\/category\/updates-announcements\/\" rel=\"category tag\">Updates & Announcements<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/ai\/' rel='post_tag'>AI<\/a><a href='https:\/\/successfulhf.com\/tag\/ai-for-human-factors\/' rel='post_tag'>AI for Human Factors<\/a><a href='https:\/\/successfulhf.com\/tag\/ai-for-ux-research\/' rel='post_tag'>AI for UX Research<\/a><a href='https:\/\/successfulhf.com\/tag\/artificial-intelligence\/' rel='post_tag'>Artificial Intelligence<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors\/' rel='post_tag'>Human Factors<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-engineering\/' rel='post_tag'>Human Factors Engineering<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices-and-ai\/' rel='post_tag'>Medical Devices and AI<\/a><a href='https:\/\/successfulhf.com\/tag\/patient-safety\/' rel='post_tag'>Patient Safety<\/a><a href='https:\/\/successfulhf.com\/tag\/suabix\/' rel='post_tag'>suabix<\/a>"},"readTime":{"min":1,"sec":59},"status":"publish","excerpt":"Suabix\u2122 by Successful Human Factors\u2122, is the first AI Human Factors Copilot for medical product teams, revolutionizing the future of healthcare and patient safety. Successful Human Factors\u2122 highlights AI integration services tailored to stakeholders needs, powered by Suabix."},{"id":20358,"link":"https:\/\/successfulhf.com\/original-insights-success-factors-of-human-factors-validation\/","name":"original-insights-success-factors-of-human-factors-validation","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/Original-Insights-Success-Factors-of-Human-Factors-Validation.png","alt":""},"title":"Original Insights:\u00a0Success Factors of Human Factors Validation in Medical Device Development","author":{"name":"Staff","link":"https:\/\/successfulhf.com\/author\/staff\/"},"date":"Dec 11, 2023","dateGMT":"2023-12-11 11:21:46","modifiedDate":"2023-12-14 17:36:29","modifiedDateGMT":"2023-12-14 17:36:29","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/\" rel=\"category tag\">Insights<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/\" rel=\"category tag\">Insights<\/a>"},"taxonomies":{"post_tag":""},"readTime":{"min":6,"sec":30},"status":"publish","excerpt":"Uncover the key success factors and best practices for optimized outcomes in your human factors validations. Contact us for personalized assistance and take a leap towards enhancing your HF strategy for unparalleled success in medical device development."},{"id":20094,"link":"https:\/\/successfulhf.com\/navigating-the-shadows-a-tale-of-innovation-plagiarism-and-the-battle-for-ethical-standards-in-human-factors-and-usability-engineering\/","name":"navigating-the-shadows-a-tale-of-innovation-plagiarism-and-the-battle-for-ethical-standards-in-human-factors-and-usability-engineering","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/dont-copy-innovate-e1700468707506.png","alt":""},"title":"Navigating the Shadows: A Tale of Innovation, Plagiarism, and the Battle for Ethical Standards in Human Factors and Usability Engineering","author":{"name":"Dr. Katia Rojas","link":"https:\/\/successfulhf.com\/author\/rojaskatiamgmail-com\/"},"date":"Nov 20, 2023","dateGMT":"2023-11-20 07:59:39","modifiedDate":"2024-02-16 17:02:27","modifiedDateGMT":"2024-02-16 17:02:27","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/discussions-trends\/\" rel=\"category tag\">Discussions & Trends<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/discussions-trends\/\" rel=\"category tag\">Discussions & Trends<\/a>"},"taxonomies":{"post_tag":""},"readTime":{"min":2,"sec":4},"status":"publish","excerpt":"\"Imitation may be the sincerest form of flattery, but originality is the mark of true innovation.\" - Unknown"},{"id":19723,"link":"https:\/\/successfulhf.com\/announcement-introducing-suite-of-human-factors-services-for-healthcare-teams\/","name":"announcement-introducing-suite-of-human-factors-services-for-healthcare-teams","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/healthcare-9-e1697210889759.png","alt":""},"title":"Announcement: Introducing Suite of Services to Support Healthcare Teams with Innovative Solutions!","author":{"name":"Staff","link":"https:\/\/successfulhf.com\/author\/staff\/"},"date":"Oct 18, 2023","dateGMT":"2023-10-18 21:04:43","modifiedDate":"2023-10-19 21:43:01","modifiedDateGMT":"2023-10-19 21:43:01","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/industry-news\/\" rel=\"category tag\">Industry News<\/a>, <a href=\"https:\/\/successfulhf.com\/category\/updates-announcements\/\" rel=\"category tag\">Updates & Announcements<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/industry-news\/\" rel=\"category tag\">Industry News<\/a> <a href=\"https:\/\/successfulhf.com\/category\/updates-announcements\/\" rel=\"category tag\">Updates & Announcements<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/combination-products\/' rel='post_tag'>Combination Products<\/a><a href='https:\/\/successfulhf.com\/tag\/factores-humanos-fda\/' rel='post_tag'>Factores Humanos FDA<\/a><a href='https:\/\/successfulhf.com\/tag\/fda-human-factors\/' rel='post_tag'>FDA Human Factors<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-consulting-services\/' rel='post_tag'>Human Factors Consulting Services<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-testing\/' rel='post_tag'>Human Factors Testing<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/usability-study\/' rel='post_tag'>Usability Study<\/a>"},"readTime":{"min":3,"sec":55},"status":"publish","excerpt":"Full spectrum of expert, industry-focused human factors & usability engineering services to drive excellence in healthcare!"},{"id":18035,"link":"https:\/\/successfulhf.com\/from-research-to-policy-exploring-precedents-of-hfes-fda-maturity-model-recommendation\/","name":"from-research-to-policy-exploring-precedents-of-hfes-fda-maturity-model-recommendation","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/2023\/06\/Watch-the-gap.png","alt":""},"title":"From Research to Policy: Exploring Precedents of HFES Maturity Framework Policy Recommendations to the FDA","author":{"name":"Dr. Katia Rojas","link":"https:\/\/successfulhf.com\/author\/rojaskatiamgmail-com\/"},"date":"Sep 24, 2023","dateGMT":"2023-09-24 23:43:18","modifiedDate":"2024-01-06 17:36:29","modifiedDateGMT":"2024-01-06 17:36:29","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/discussions-trends\/\" rel=\"category tag\">Discussions & Trends<\/a>, <a href=\"https:\/\/successfulhf.com\/category\/industry-news\/\" rel=\"category tag\">Industry News<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/discussions-trends\/\" rel=\"category tag\">Discussions & Trends<\/a> <a href=\"https:\/\/successfulhf.com\/category\/industry-news\/\" rel=\"category tag\">Industry News<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/collaboration\/' rel='post_tag'>Collaboration<\/a><a href='https:\/\/successfulhf.com\/tag\/fda\/' rel='post_tag'>FDA<\/a><a href='https:\/\/successfulhf.com\/tag\/hfe\/' rel='post_tag'>HFE<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-engineering\/' rel='post_tag'>Human Factors Engineering<\/a><a href='https:\/\/successfulhf.com\/tag\/innovation\/' rel='post_tag'>Innovation<\/a><a href='https:\/\/successfulhf.com\/tag\/maturity\/' rel='post_tag'>Maturity<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/medtech\/' rel='post_tag'>MedTech<\/a><a href='https:\/\/successfulhf.com\/tag\/quality-assurance\/' rel='post_tag'>Quality Assurance<\/a>"},"readTime":{"min":13,"sec":24},"status":"publish","excerpt":"\"The opinion of 10,000 men is of no value if none of them know anything about the subject.\" (Marcus Aurelius)"},{"id":16349,"link":"https:\/\/successfulhf.com\/suabix-healthcare-ai-powered-human-factors-trademark\/","name":"suabix-healthcare-ai-powered-human-factors-trademark","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/Untitled-design.png","alt":""},"title":"Suabix: AI-Powered Human Factors Innovation, Trademark Filed","author":{"name":"Staff","link":"https:\/\/successfulhf.com\/author\/staff\/"},"date":"Sep 15, 2023","dateGMT":"2023-09-15 00:11:57","modifiedDate":"2024-07-25 17:38:12","modifiedDateGMT":"2024-07-25 17:38:12","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/announcements\/\" rel=\"category tag\">Announcements<\/a>, <a href=\"https:\/\/successfulhf.com\/category\/updates\/\" rel=\"category tag\">Updates<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/announcements\/\" rel=\"category tag\">Announcements<\/a> <a href=\"https:\/\/successfulhf.com\/category\/updates\/\" rel=\"category tag\">Updates<\/a>"},"taxonomies":{"post_tag":""},"readTime":{"min":2,"sec":32},"status":"publish","excerpt":"Artificial Intelligence for Human Factors and Usability Engineering Optimization"},{"id":14977,"link":"https:\/\/successfulhf.com\/introducing-suabix-by-successful-human-factors\/","name":"introducing-suabix-by-successful-human-factors","thumbnail":{"url":false,"alt":false},"title":"Introducing Suabix\u2122 by Successful Human Factors\u2122 - First AI Solution to Optimize and Standardize Human Factors in Medical Device Design","author":{"name":"Staff","link":"https:\/\/successfulhf.com\/author\/staff\/"},"date":"Aug 22, 2023","dateGMT":"2023-08-22 15:11:24","modifiedDate":"2023-08-22 15:11:24","modifiedDateGMT":"2023-08-22 15:11:24","commentCount":"0","commentStatus":"closed","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/updates\/\" rel=\"category tag\">Updates<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/updates\/\" rel=\"category tag\">Updates<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/ai\/' rel='post_tag'>AI<\/a><a href='https:\/\/successfulhf.com\/tag\/biotech\/' rel='post_tag'>Biotech<\/a><a href='https:\/\/successfulhf.com\/tag\/combination-products\/' rel='post_tag'>Combination Products<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-testing\/' rel='post_tag'>Human Factors Testing<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-validation\/' rel='post_tag'>Human Factors Validation<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/medtech\/' rel='post_tag'>MedTech<\/a><a href='https:\/\/successfulhf.com\/tag\/usability-testing\/' rel='post_tag'>Usability Testing<\/a>"},"readTime":{"min":2,"sec":41},"status":"publish","excerpt":"New York-based Successful Human Factors\u2122 introduces Suabix\u2122, an innovative AI-powered platform that integrates human factors into medical device design, addressing industry challenges and fostering excellence. Suabix\u2122 offers automated usability testing, intelligent reporting, expert guidance, and seamless integration, benefiting all stakeholders and accelerating time-to-market. Early access opportunities are available for select participants seeking to enhance their product experiences."},{"id":17332,"link":"https:\/\/successfulhf.com\/understanding-the-key-success-factors-in-tools-methodology-within-the-successful-human-factors-2-0-framework-part-i\/","name":"understanding-the-key-success-factors-in-tools-methodology-within-the-successful-human-factors-2-0-framework-part-i","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/depict-product-development-professionals-consumed-with-iterative-and-manual-tasks-e1705512694522.png","alt":""},"title":"Understanding the Key Success Factors in \u2018Tools & Methodology\u2019 within the Successful Human Factors 2.0 Framework (Part I)","author":{"name":"Dr. Katia Rojas","link":"https:\/\/successfulhf.com\/author\/rojaskatiamgmail-com\/"},"date":"Jul 14, 2023","dateGMT":"2023-07-14 15:29:37","modifiedDate":"2024-01-17 17:31:53","modifiedDateGMT":"2024-01-17 17:31:53","commentCount":"0","commentStatus":"open","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/tools-methodology\/' rel='post_tag'>Tools & Methodology<\/a>"},"readTime":{"min":6,"sec":12},"status":"publish","excerpt":""},{"id":16675,"link":"https:\/\/successfulhf.com\/redefining-human-factors-validations-strategies-to-increase-maturity-for-successful-human-factors-in-medical-devices-and-combination-products\/","name":"redefining-human-factors-validations-strategies-to-increase-maturity-for-successful-human-factors-in-medical-devices-and-combination-products","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/2023\/06\/doctors-seminar-background-1-scaled.jpeg","alt":""},"title":"Redefining Human Factors Validations: Strategies to Increase Maturity for Successful Human Factors in Medical Devices and Combination Products","author":{"name":"Dr. Katia Rojas","link":"https:\/\/successfulhf.com\/author\/rojaskatiamgmail-com\/"},"date":"Jun 30, 2023","dateGMT":"2023-06-30 19:31:33","modifiedDate":"2024-02-02 22:44:58","modifiedDateGMT":"2024-02-02 22:44:58","commentCount":"0","commentStatus":"closed","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/discussions-trends\/\" rel=\"category tag\">Discussions & Trends<\/a>, <a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/discussions-trends\/\" rel=\"category tag\">Discussions & Trends<\/a> <a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/fda\/' rel='post_tag'>FDA<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-strategy\/' rel='post_tag'>Human Factors Strategy<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-testing\/' rel='post_tag'>Human Factors Testing<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-validations\/' rel='post_tag'>Human Factors Validations<\/a><a href='https:\/\/successfulhf.com\/tag\/maturity\/' rel='post_tag'>Maturity<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/medtech\/' rel='post_tag'>MedTech<\/a><a href='https:\/\/successfulhf.com\/tag\/project-success\/' rel='post_tag'>Project Success<\/a><a href='https:\/\/successfulhf.com\/tag\/quality-assurance\/' rel='post_tag'>Quality Assurance<\/a><a href='https:\/\/successfulhf.com\/tag\/shf-2-0\/' rel='post_tag'>SHF 2.0<\/a><a href='https:\/\/successfulhf.com\/tag\/strategies\/' rel='post_tag'>Strategies<\/a><a href='https:\/\/successfulhf.com\/tag\/usability-testing\/' rel='post_tag'>Usability Testing<\/a>"},"readTime":{"min":8,"sec":4},"status":"publish","excerpt":""},{"id":16246,"link":"https:\/\/successfulhf.com\/anticipating-common-challenges-in-medical-device-human-factors-validation-projects-with-the-shf-2-0-framework-a-strategy-implementation-approach\/","name":"anticipating-common-challenges-in-medical-device-human-factors-validation-projects-with-the-shf-2-0-framework-a-strategy-implementation-approach","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/2023\/06\/failed-hf-validation.jpg","alt":""},"title":"Leveraging the SHF 2.0 Framework to Overcome Common Challenges in Human Factors Validation Projects: A Strategy & Implementation Approach","author":{"name":"Dr. Katia Rojas","link":"https:\/\/successfulhf.com\/author\/rojaskatiamgmail-com\/"},"date":"Jun 13, 2023","dateGMT":"2023-06-13 18:29:02","modifiedDate":"2023-07-28 20:57:44","modifiedDateGMT":"2023-07-28 20:57:44","commentCount":"0","commentStatus":"closed","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/human-factors-strategy\/' rel='post_tag'>Human Factors Strategy<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-testing\/' rel='post_tag'>Human Factors Testing<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/medtech\/' rel='post_tag'>MedTech<\/a><a href='https:\/\/successfulhf.com\/tag\/project-success\/' rel='post_tag'>Project Success<\/a><a href='https:\/\/successfulhf.com\/tag\/shf-2-0\/' rel='post_tag'>SHF 2.0<\/a><a href='https:\/\/successfulhf.com\/tag\/usability-testing\/' rel='post_tag'>Usability Testing<\/a>"},"readTime":{"min":5,"sec":13},"status":"publish","excerpt":""},{"id":16041,"link":"https:\/\/successfulhf.com\/research-urges-standardization-of-fdas-human-factors-engineering-practices-within-the-medical-device-industry\/","name":"research-urges-standardization-of-fdas-human-factors-engineering-practices-within-the-medical-device-industry","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/2023\/05\/Framework-SHF-2.0-May-2023.png","alt":""},"title":"Research Urges Standardization of FDA's Human Factors Engineering Practices Within the Medical Device Industry","author":{"name":"Dr. Katia Rojas","link":"https:\/\/successfulhf.com\/author\/rojaskatiamgmail-com\/"},"date":"Jun 9, 2023","dateGMT":"2023-06-09 19:52:41","modifiedDate":"2024-06-30 23:58:35","modifiedDateGMT":"2024-06-30 23:58:35","commentCount":"0","commentStatus":"closed","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/updates-announcements\/\" rel=\"category tag\">Updates & Announcements<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/updates-announcements\/\" rel=\"category tag\">Updates & Announcements<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/fda\/' rel='post_tag'>FDA<\/a><a href='https:\/\/successfulhf.com\/tag\/hf-project-failure\/' rel='post_tag'>HF Project Failure<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/medtech\/' rel='post_tag'>MedTech<\/a><a href='https:\/\/successfulhf.com\/tag\/project-success\/' rel='post_tag'>Project Success<\/a><a href='https:\/\/successfulhf.com\/tag\/shf-2-0\/' rel='post_tag'>SHF 2.0<\/a><a href='https:\/\/successfulhf.com\/tag\/usability-testing\/' rel='post_tag'>Usability Testing<\/a>"},"readTime":{"min":3,"sec":48},"status":"publish","excerpt":""},{"id":14250,"link":"https:\/\/successfulhf.com\/use-cases\/","name":"use-cases","thumbnail":{"url":"https:\/\/successfulhf.com\/wp-content\/uploads\/2023\/05\/2-1.png","alt":""},"title":"SHF 2.0 Framework - Use Cases","author":{"name":"Staff","link":"https:\/\/successfulhf.com\/author\/staff\/"},"date":"May 17, 2023","dateGMT":"2023-05-17 14:43:28","modifiedDate":"2024-01-12 17:17:33","modifiedDateGMT":"2024-01-12 17:17:33","commentCount":"0","commentStatus":"closed","categories":{"coma":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>","space":"<a href=\"https:\/\/successfulhf.com\/category\/insights\/strategy-implementation\/\" rel=\"category tag\">HFE Strategy & Implementation<\/a>"},"taxonomies":{"post_tag":"<a href='https:\/\/successfulhf.com\/tag\/benchmark\/' rel='post_tag'>Benchmark<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-strategy\/' rel='post_tag'>Human Factors Strategy<\/a><a href='https:\/\/successfulhf.com\/tag\/human-factors-validations\/' rel='post_tag'>Human Factors Validations<\/a><a href='https:\/\/successfulhf.com\/tag\/medical-devices\/' rel='post_tag'>Medical Devices<\/a><a href='https:\/\/successfulhf.com\/tag\/medtech\/' rel='post_tag'>MedTech<\/a><a href='https:\/\/successfulhf.com\/tag\/shf-2-0\/' rel='post_tag'>SHF 2.0<\/a>"},"readTime":{"min":3,"sec":53},"status":"publish","excerpt":""}]
